https://sputniknews.in/20230203/indian-company-recalls-eyedrops-from-us-market-over-dangerous-bacteria-750333.html
Indian Company Recalls Eyedrops From US Market Over Dangerous Bacteria
Indian Company Recalls Eyedrops From US Market Over Dangerous Bacteria
Sputnik India
Global Pharma, an Indian pharmaceutical company, has recalled its eyedrop, EzriCare Artificial Tears, from the US market after it was linked to a drug-resistant bacteria strain that has caused at least one person's death.
2023-02-03T18:37+0530
2023-02-03T18:37+0530
2023-02-03T18:37+0530
us
india
https://cdn1.img.sputniknews.in/img/07e7/02/03/751897_0:43:695:433_1920x0_80_0_0_d8a1e02cc85fb005eac4588da06e9266.jpg
Global Pharma, an Indian pharmaceutical company, has recalled its EzriCare Artificial Tears from the US market after the eyedrops were suspected of containing a drug-resistant bacteria strain.After this, Global Pharma said that it was recalling the eyedrops "out of an abundance of caution" on Friday.The US Centers for Disease Control and Prevention (CDC) has announced that it is currently testing unopened bottles of the eyedrops manufactured by the Chennai-based company. Meanwhile, the US Food and Drug Administration (FDA) said it has moved to restrict imports of products made by the company.Last month, hospitals saw a rise in cases of Pseudomonas aeruginosa and noted that people were using artificial teardrops. Most of these patients reported using EzriCare's drops. The eye drops are preservative-free, meaning they don't have ingredients to prevent bacterial growth.This also suggests that the bacteria in the open bottles of Ezricare could have come from contamination either during use or during the manufacturing process, the CDC suggested. How Dangerous is Pseudomonas Aeruginosa?Pseudomonas aeruginosa can cause infections in the blood, lungs, or wounds, and the germ has been proving tougher to treat in recent times because of antibiotic resistance, Insider.com reported.The CDC says that Pseudomonas aeruginosa is usually spread in healthcare settings and is increasingly difficult to treat because of antibiotic resistance.It caused more than 32,000 infections in hospitalized patients and about 2,700 deaths in the US in 2017.
https://sputniknews.in/20230124/who-calls-for-action-against-cough-syrups-linked-to-over-300-child-deaths-worldwide-607741.html
us
india
Sputnik India
feedback.hindi@sputniknews.com
+74956456601
MIA „Rossiya Segodnya“
2023
Deexa Khanduri
https://cdn1.img.sputniknews.in/img/07e6/0c/13/138923_52:0:533:481_100x100_80_0_0_cadf23d341691fc65ff2b22fd1afe584.jpg
Deexa Khanduri
https://cdn1.img.sputniknews.in/img/07e6/0c/13/138923_52:0:533:481_100x100_80_0_0_cadf23d341691fc65ff2b22fd1afe584.jpg
News
en_IN
Sputnik India
feedback.hindi@sputniknews.com
+74956456601
MIA „Rossiya Segodnya“
Sputnik India
feedback.hindi@sputniknews.com
+74956456601
MIA „Rossiya Segodnya“
Deexa Khanduri
https://cdn1.img.sputniknews.in/img/07e6/0c/13/138923_52:0:533:481_100x100_80_0_0_cadf23d341691fc65ff2b22fd1afe584.jpg
global pharma, global pharma healthcare, ezricare, ezricare drop, pseudomonas aeruginosa, pseudomonas aeruginosa eye infection, eye infection, indian company, indian pharmaceutical, how dangerous is pseudomonas aeruginosa
global pharma, global pharma healthcare, ezricare, ezricare drop, pseudomonas aeruginosa, pseudomonas aeruginosa eye infection, eye infection, indian company, indian pharmaceutical, how dangerous is pseudomonas aeruginosa
Indian Company Recalls Eyedrops From US Market Over Dangerous Bacteria
Deexa Khanduri
Sputnik correspondent
Indian pharmaceutical firms have had a tough time of late. Last year in two separate incidents in Gambia and Uzbekistan, children died after taking Indian-made cough syrups.
Global Pharma, an Indian pharmaceutical company, has recalled its EzriCare Artificial Tears from the US market after the eyedrops were suspected of containing a drug-resistant bacteria strain.
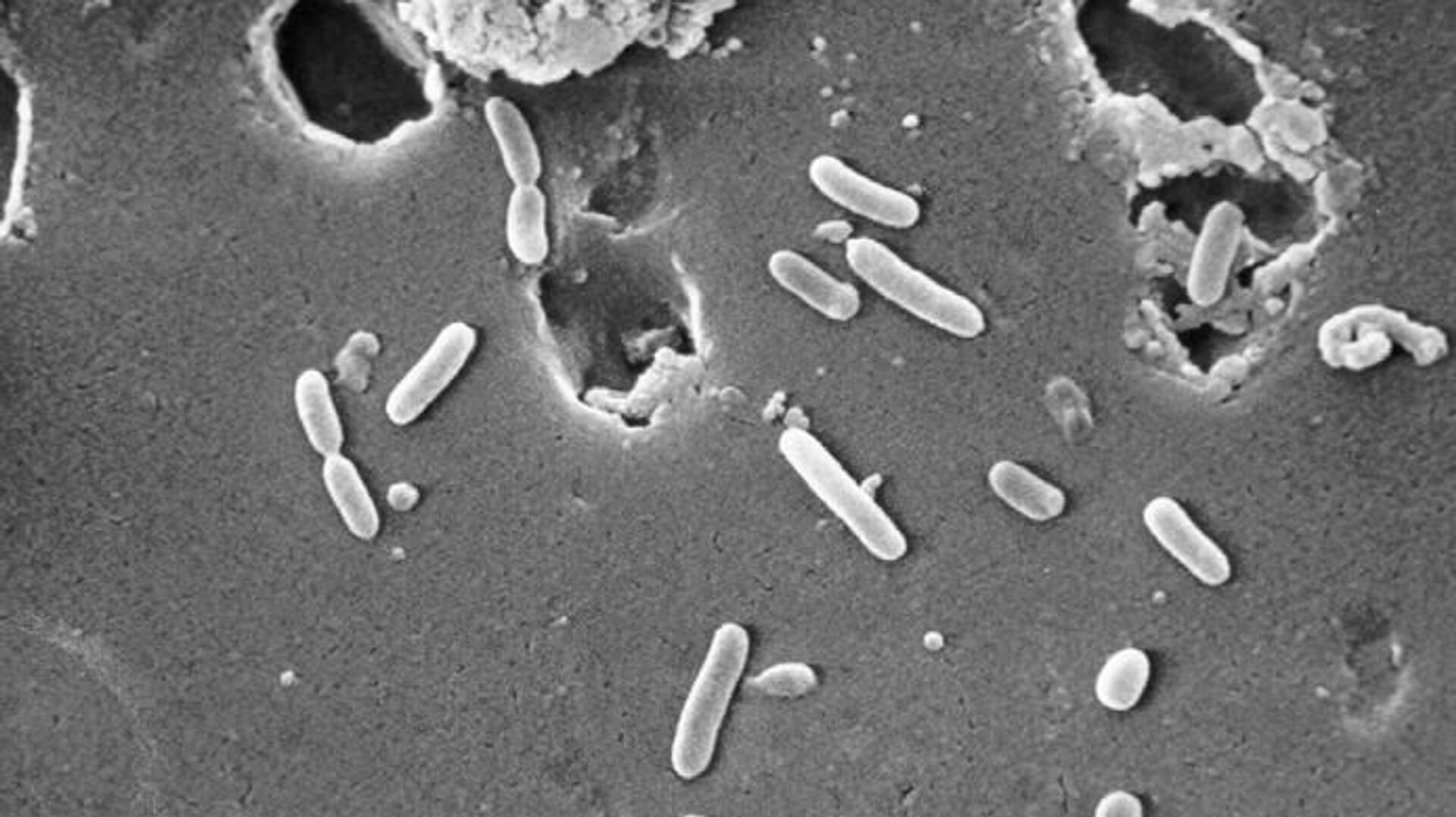
US health officials raised alarm over an unprecedented outbreak of Pseudomonas aeruginosa, which allegedly affected at least 55 people across a dozen US states and caused at least one death and five cases of vision loss.
After this, Global Pharma said that it was recalling the eyedrops "out of an abundance of caution" on Friday.
The US Centers for Disease Control and Prevention (CDC) has announced that it is currently testing unopened bottles of the eyedrops manufactured by the Chennai-based company. Meanwhile, the US Food and Drug Administration (FDA) said it has moved to restrict imports of products made by the company.
Last month, hospitals saw a rise in cases of Pseudomonas aeruginosa and noted that people were using artificial teardrops. Most of these patients reported using EzriCare's drops.
The eye drops are preservative-free, meaning they don't have ingredients to prevent bacterial growth.
This also suggests that the bacteria in the open bottles of Ezricare could have come from contamination either during use or during the manufacturing process, the CDC suggested.
How Dangerous is Pseudomonas Aeruginosa?
Pseudomonas aeruginosa can cause infections in the blood, lungs, or wounds, and the germ has been proving tougher to treat in recent times because of antibiotic resistance, Insider.com reported.
The CDC says that Pseudomonas aeruginosa is usually spread in healthcare settings and is increasingly difficult to treat because of antibiotic resistance.
It caused more than 32,000 infections in hospitalized patients and about 2,700 deaths in the US in 2017.